For all those interested in purchasing our books from outside the United States, we now have added two shipping options, one for Canada and another for most everywhere else. You will see these options at the checkout portion of the shopping cart as shipping options. We have set prices only for 1 or 2 books (which are the same price). If you would like to purchase more than 2 books or have additional questions, please email us at orders@pointinstitute.org
Ebook versions are NOT yet available.
Author Archives: pointadmin
Prenatal Standard Now Available for Download
Our last Standard publication focusing on Nutrition and Supplementation during pregnancy is now available for download in our Resource Section. Prenatal Nutrition: The Role of Diet and Supplementation (Vol. 11 no 1). A review covering the unique dietary needs and recommendations for pregnant (or soon to be pregnant) women. This covers dietary patterns, macronutrients, vitamins, minerals, key support nutrients and even probiotic recommendations. This discussion covers basic nutrient mechanisms as well as genomic (and epigenetic) influences of nutrients. Also covered are nuances between different forms of supplemental folates, iron and vitamin B12, some of which may be of interest to the clinician making specific recommendations.
You can download your copy here: https://www.pointinstitute.org/the-standard/
Re-assessing the Notion of “Pregnenolone Steal”
Re-assessing the Notion of “Pregnenolone Steal”
When clinicians measure salivary cortisol and DHEA (DHEA-S) to assess stress and HPA axis function, it is common to find DHEA levels below the reference range in a number of individuals. A common explanation for the depletion of DHEA and other hormones (e.g., progesterone, testosterone) due to chronic stress is the phenomenon known as “pregnenolone steal.” This notion basically states that since all steroid hormones use pregnenolone (derived from cholesterol) as a precursor, the elevated secretion of cortisol caused by acute or chronic stress will inevitably result in less available pregnenolone to serve as a precursor for the production of DHEA and other down-stream hormones. In other words, according to this theory, the need for cortisol synthesis “steals” pregnenolone away from other hormone pathways, reducing the potential synthesis and secretion of other necessary hormones, resulting in some of the pathophysiological changes related to stress.
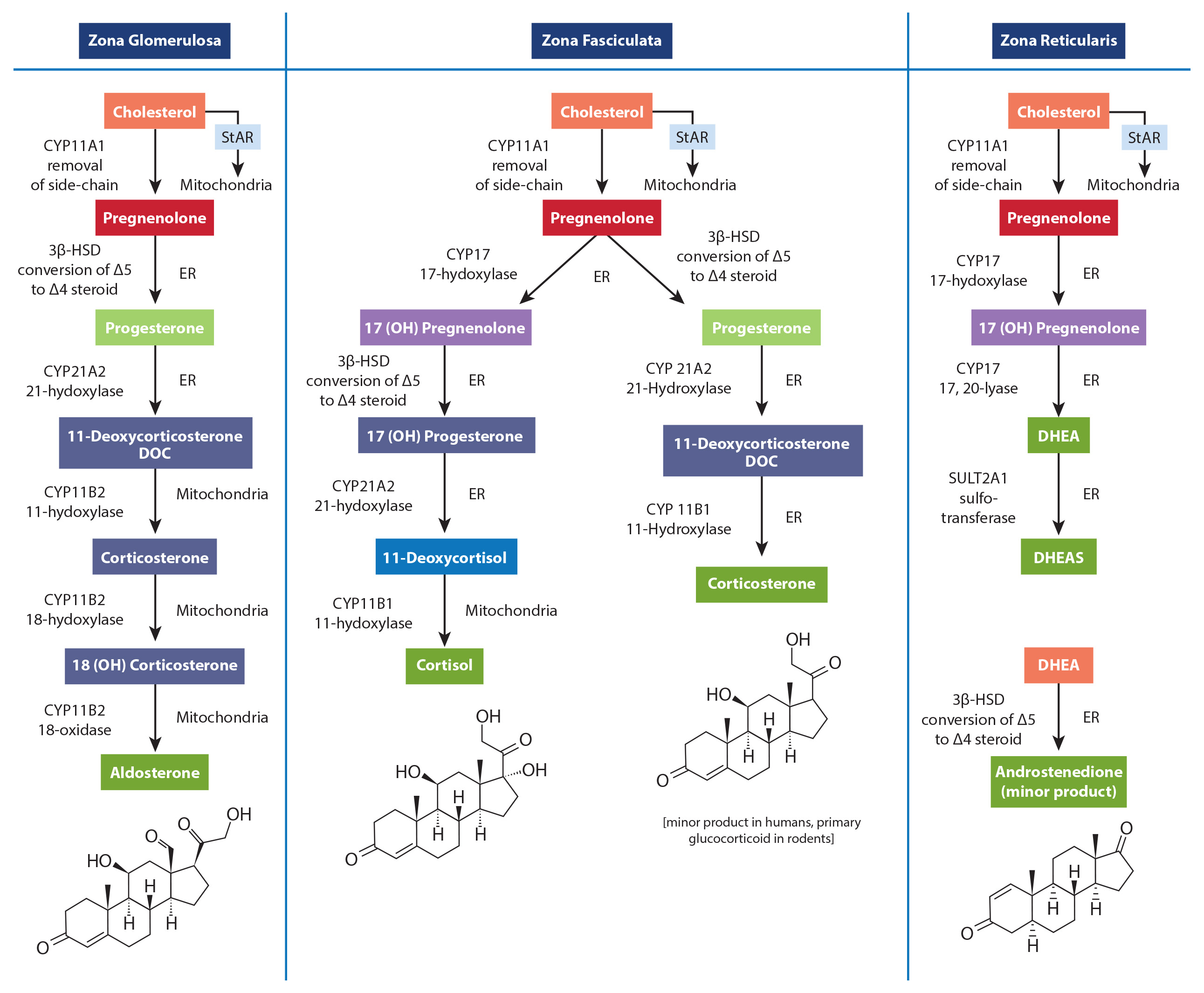
While a rise in cortisol levels and a concomitant drop in DHEA is one of the clinical characteristics of early and mid-stage chronic stress progression, this phenomenon is not caused by diminished adrenal pregnenolone availability or “pregnenolone steal.” The most obvious reason is the fact that the conversion of cholesterol to pregnenolone occurs in the mitochondria of each respective adrenal cortex cell type that is responsible for producing these hormones. Simply put, there is no known adrenal pool of pregnenolone for one cell to steal away from another, and no known mechanism has been described that could facilitate the transfer of pregnenolone between the mitochondria of different cells (in this case, from the mitochondria of cells within the zona reticularis to those within the zona fasciculata). Unfortunately, the most common figures used to teach steroidogenesis show a common pathway and typically do not specify the differential regulation of available enzymes between different steroidogenic tissues. This leads many to incorrectly assume there is a single “pool” of pregnenolone available for all steroid hormone synthesis within the adrenal. A much better way to teach this is to show the different enzymes available to each cell within the adrenal cortex, showing that each is capable of converting cholesterol to pregnenolone; then to the various needed hormones. This is a figure from the new book- that shows a better way to teach this that avoids showing a single “pool” of pregnenolone for all down-stream hormones.
In addition, while the ACTH-driven adrenal synthesis of cortisol is orders of magnitude higher than that of DHEA, and fluctuates radically within a 24-hour period, the overall synthesis of DHEA and DHEA-S is 500-1000 higher than that of cortisol. Therefore, even if cortisol and DHEA production shared a common pool of pregnenolone (which they do not), the amount of pregnenolone used for cortisol production (even when elevated) would have very little effect on the production of DHEA(S). Finally, as decades of steroidogenesis research has shown, the control of adrenal hormone output is regulated mostly by cell-specific enzyme concentrations and external signals coming from outside the adrenal gland (See our latest book for specifics).
What, then, does this mean in relation to cortisol and DHEA output which, when measured, appears to confirm this phenomenon? What about the role of oral pregnenolone therapy for supporting adrenal DHEA production? Well, it’s a bit complicated. While HPA axis stress and subsequent cortisol synthesis and secretion may be related to the acceleration of reduced DHEA production (i.e., a stress-induced down-regulation of DHEA), this relationship is facilitated by regulatory processes (e.g., feedback inhibitions, receptor signaling, genomic regulation of enzymes, etc.), not an intra-adrenal depletion of pregnenolone as a precursor to downstream hormones. For instance, experimentally-induced hyperglycemia and hyperinsulinemia has been shown to affect DHEA and androstenedione production in human subjects.[1],[2] In one study of poorly-controlled type 2 diabetic subjects with elevated cortisol and low DHEA levels, the enzyme necessary for DHEA formation in the zona reticularis (17,20 lyase) was shown to limit the production of DHEA. The enzyme activity was corrected (along with near normalization of cortisol, DHEA and DHEA-S levels) after six months of diet or pharmacotherapy to improve blood glucose control.[3] Additionally, cell-culture studies suggest that under inflammatory stress (IL-4 and other cytokines), the zona reticularis will down-regulate DHEA production when ACTH is present.[4],[5]These and many other factors (e.g., aging) are likely the driving influences affecting the dynamic relationship between cortisol (activated by the HPA axis) and measured DHEA and/or DHEA-S levels.
By re-assessing the specific mechanisms that drive the stress-related changes in adrenal hormone output, and moving away from older and incorrect explanations, we are able to seek (and perhaps address) the various signals that are actually responsible for modulating adrenal hormone secretion during the progression of chronic stress.
If you are interested in learning more about this subject and how oral pregnenolone and DHEA may improve outcomes in subjects with stress-related dysfunctions, please consider getting our newest book: The Role of Stress and the HPA Axis in Chronic Disease Management.
[1] Boudou P, Sobngwi E, Ibrahim F et al. Hyperglycaemia acutely decreases circulating dehydroepiandrosterone levels in healthy men. Clin Endocrinol (Oxf). 2006 Jan;64(1):46-52.
[2] Vásárhelyi B, Bencsik P, Treszl A, et al. The effect of physiologic hyperinsulinemia during an oral glucose tolerance test on the levels of dehydroepiandrosterone (DHEA) and its sulfate (DHEAS) in healthy young adults born with low and with normal birth weight. Endocr J. 2003 Dec;50(6):689-95.
[3] Ueshiba H, Shimizu Y, Hiroi N et al. Decreased steroidogenic enzyme 17,20-lyase and increased 17-hydroxylase activities in type 2 diabetes mellitus. Eur J Endocrinol. 2002 Mar;146(3):375-80.
[4] Woods AM, Judd AM. Interleukin-4 increases cortisol release and decreases adrenal androgen release from bovine adrenal cells. Domest Anim Endocrinol. 2008 May;34(4):372-82
[5] Woods AM, McIlmoil CJ, Rankin EN. Et al. Leukemia inhibitory factor protein and receptors are expressed in the bovine adrenal cortex and increase cortisol and decrease adrenal androgen release. Domest Anim Endocrinol. 2008 Aug;35(2):217-30
Is it really “Adrenal Fatigue” or HPA axis dysfunction?
Is it “Adrenal Fatigue”?
Reassessing the Nomenclature of HPA Axis Dysfunction
Sometimes, when we endeavor to understand and describe complicated medical topics, there is a temptation to find a simple explanation to cut through the complexity. These explanations can help bridge the knowledge gap for a while, but as our knowledge grows, they lose some of their original usefulness (e.g., the notion of “good” and “bad” cholesterol). In some cases, those over-simplified explanations actually become a hindrance to helping clinicians and patients understand the important mechanisms and solutions related to chronic conditions. The use of terms like “adrenal fatigue” and “adrenal exhaustion” to summarize the complex dysfunctions related to the stress response is one such explanation. Though these terms have helped dispel the notion that only extreme issues related to adrenal function (Addison’s disease or Cushing’s disease) are of clinical importance, and have become surrogate descriptions for stress-related outcomes, they should now be replaced by more accurate and medically appropriate terms, like HPA axis dysfunction, adrenal insufficiency, or where applicable, hypocortisolism.
While it is true that the most common laboratory method to assess the function of the HPA axis is through the measurement of hormones secreted by the adrenal glands, primarily cortisol and DHEA(S), the mechanisms that control the level of these hormones resides mostly outside of the adrenal gland. Low cortisol and DHEA(S) levels may indeed be related to chronic stress, but as a result of HPA axis adaption (down-regulation) to protect tissues from excess cortisol, have little to do with the inherent capability of the adrenal gland to produce these hormones (see adrenal insufficiency below). While many clinicians (and laboratories) still refer to this as “testing the adrenals,” it is much more accurate to say that such testing is assessing the status of the HPA axis using adrenal hormone measurements as surrogate markers. So, why does this nomenclature reassessment matter?
First of all, using descriptive and accurate terms helps clinicians and patients better understanding the pathophysiology caused by stress and the stress response system. In most cases, issues related to perceived stress, glycemic control, circadian rhythm, cortisol feedback control (in the hypothalamus and/or pituitary), inflammatory signaling, or tissue-specific glucocorticoid effects will have much more to do with a treatment protocol than direct support of adrenal function. For instance, many adaptogenic herbs and nutrients that were once thought to function primarily by supporting adrenal function have been shown to have mechanism that modulate non-adrenal HPA axis or glucocorticoid signaling functions. Related to this is the ability of the clinician to interface appropriately with the vast amount of literature that describes patient outcomes related to stress and HPA axis function. The term “adrenal fatigue” is virtually absent from the peer-reviewed literature and has even caused the Endocrine Society to warn the public against the diagnostic “myth” of adrenal fatigue and to cast suspicion upon clinicians using such terms. While I generally agree with the Endocrine Society that the term “adrenal fatigue” is problematic, I do not agree with them that there is little evidence to connect chronic stress with adverse health outcomes, or that testing adrenal hormone output is of no value beyond diagnosing extreme adrenal disease conditions.
A
n increasing body of research links a variety of chronic dysfunctions with specific patterns of adrenal hormone output (basal or provoked). By avoiding the use of oversimplified (and incorrect) terminology to describe these relationships and instead choosing more appropriate descriptive terms, the clinician will enhance the credibility of this important phenomenon and be better equipped to incorporate therapies that address the complexity of the whole stress response system.
What are More Appropriate Terms?
HPA Axis Dysfunction (or Maladaption): This term is much more appropriate to describe the many consequences that link stress (allostasis) with the myriad of measurable negative outcomes related to the stress response. The majority of these outcomes can be linked in some manner to processes controlled by the HPA axis. Alternatively, some refer to these as “disorders of the stress system” or the “consequences of the maladaption to stress.”
Hypocortisolism: This is the most descriptive term to use when measured cortisol is well below the laboratory reference range. Still, it is a relative term and does not necessarily implicate dysfunction or “fatigue” of the adrenal gland. Extreme hypocortisolism is associated with Addison’s disease and other forms of primary and secondary adrenal insufficiency. Reduced HPA axis function resulting in low cortisol levels is common in PTSD, fibromyalgia, chronic fatigue syndrome, certain affective disorders, and individuals with high psychosocial “burnout”. Other specific terms for different stress-related HPA axis phenomena include hypercortisolism, loss of HPA circadian function, and low circulating DHEA(S).
Adrenal Insufficiency: This is a clinical manifestation that results in a deficient production or action of glucocorticoids, a condition that has potential life-threatening consequences. Primary adrenal insufficiency (i.e., Addison’s disease) describes diseases intrinsic to the adrenal cortex primarily caused by autoimmune adrenalitis. Secondary adrenal insufficiency relates to insufficient pituitary ACTH or intrinsic defects in the adrenal responsiveness to ACTH. Tertiary adrenal insufficiency results from impaired synthesis of hypothalamic CRH or AVP. The most common cause of tertiary adrenal insufficiency is the chronic use of exogenous glucocorticoids (pharmacotherapy), leading to the suppression of hypothalamic secretions of CRH. True adrenal insufficiency will almost always require hydrocortisone replacement therapy (often life-long). For a complete review of the etiology, pathophysiology, clinical presentation, diagnosis and treatment approaches to adrenal insufficiency, see Charmandari, et al.[i]
[i] Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet. 2014 Jun 21;383(9935):2152-67.
Which is more regulated – Dietary supplements or the media declaring them unregulated?
According to the prevailing mantra, repeated in nearly every major media format, dietary supplements are completely (and shockingly) unregulated. We have been told that products can be made with no oversight, labels need not accurately disclose the contents of the product, and that outrageous claims of miracle cures are permitted without proof; furthermore, they claim that FDA has no power to do anything about it. The truth is, none of these characterizations are even remotely factual- no matter how often they are repeated.
The latest and loudest critique of the dietary supplement world has come from HBO’s John Oliver, where he ridiculed Dr. Oz’s congressional testimony on his “Last Week Tonight” show, before “exposing” the unregulated dietary supplement industry and the lawmakers he deemed responsible for allowing its deregulation. Whether or not you believe Dr. Oz is good or bad for the supplement industry (or the medical world in general), the ignorance of dietary supplement regulations displayed by Oliver was simply shocking and should be defined as a form of media malpractice. What Oliver has really exposed is a shockingly unregulated agenda-driven media industry; where humor, rather than truth, feeds the bottom line.
Of course, HBO is not the only offender. Nearly every medical journal editor or medical writer for news outlets permits similar statements to be printed without qualification. Even positive stories about dietary supplements include such statements. When Time magazine did a piece on the use of herbal medicine in the Cleveland Clinic, they included this gem in passing: “The FDA doesn’t regulate herbs and supplements.” A statement obviously written by someone who has never endured a 3-week FDA inspection of a dietary supplement manufacturing facility or read any of the hundreds of warning letters sent by FDA to supplement company owners; or for that matter, has taken the time to peruse the hundreds of pages of guidelines outlining how FDA regulates the dietary supplement industry.
But there appears to be something more insidious going on of late. For years we have had to deal with these unwarranted statements, usually tagged on to the latest adverse event report or “failed” vitamin study (see my blog on editorial bias in research media). However, over the past several years there seems to be an increasing emphasis in these statements targeting DSHEA (the Dietary Supplement Health Education Act) and those in congress who believe it to be a sufficient framework to protect the American people from harm while allow appropriate access to dietary supplements. Oliver went out of his way to point out that it was the massive amount of money used to lobby congress that has led to the shocking lack of regulation. Again, the unregulated staffers on Oliver’s HBO show must have been short on time to do some fact checking, so we will help them out.
According to Opensecrets.org (the same source used by Oliver) the dietary supplement industry’s lobbying dollars reached their peak in 2013 at $3.6 million. How does this compare with the pharmaceutical industry? Well in 2013, the pharmaceutical industry spent over $140 million lobbying congress (the highest amount of all industry sectors). If lobbying dollars equates to deregulation, as Oliver clearly concludes, then he and HBO must have a mini-series in the works exposing Big Pharma. And these numbers pale in comparison to the promotional dollars used by pharmaceutical companies to lobby doctors, insurance companies and consumers. In 2012, nearly $15 billion dollars was spent detailing doctors, including another $5 billion in free samples. When you total all the promotional dollars, including over $3 billion in direct to consumer marketing, the pharmaceutical industry spent over $27 billion in 2012 (an amount equaling 85% of the total revenues of all dietary supplements that same year). Remember that the amount of money spent in advertising by pharmaceutical companies is mostly spent through the same medical journals and media outlets claiming dietary supplements are unregulated- a coincidence, perhaps.
This is such an important topic that when putting together our last book (Supplementing Dietary Nutrients- A Guide for Healthcare Professionals), we specifically wanted to tackle this issue head-on. Not only is there a large chapter on the nuances of dietary supplement quality control and regulatory issues, we were able to reprint the HerbalGram article “Myths of an Unregulated Industry Dispelled” in its entirety (thanks American Botanical Council). This is a must-read for anyone who questions whether FDA has the ability or authority to properly regulate the dietary supplement industry or anybody recommending the use of dietary supplements to others.
Like all regulated industries, the dietary supplement world has companies and rogue players at its margin. Nearly all of the issues related to dietary supplement safety have come from these groups- mostly in the form of products that contain illegal drugs masquerading as dietary supplements for weight loss, sexual enhancement or sports performance. The responsible majority of dietary supplement companies would like to see FDA use its authority to remove these players from the market, an authority given to them by congress through DSHEA. So the next time you read an article declaring that FDA has no ability to regulate herbs or dietary supplements, you ought to consider asking which is more regulated: the dietary supplement industry or the media declaring it unregulated.
Get our new book: Supplementing Dietary Nutrients- A Guide for Healthcare Professionals Today.
Available for Healthcare Professionals at the Lifestyle Matrix Resource Center or directly from our site here.
If you want to know when Dr. Guilliams’ posts future blogs or when a new whitepaper is available, “Like” us on our Facebook page.
To get Dr. Guilliams’ earlier book, The Original Prescription– you can purchase directly from the Point Institute , The Lifestyle Matrix Resource Center (professional discounts available), or Amazon.
And don’t forget to sign up for our blog at the bottom of this page!
Isomers, Enantiomers and Activated Forms – Part 2: Folic Acid and 5-MTHF: What is the Real Difference Clinically?
Those who have been following the trends over the past several years know that many functional medicine clinicians have been promoting the use of 5-methyltetrahydrofolate (5-MTHF) in place of folic acid; especially in patients with a known genetic predisposition for reduced folate methylation. It is quite common to hear speaker after speaker suggest that the use of 5-MTHF is necessary for clinical benefit and that the use of folic acid is useless or even harmful. But are these statements based on reliable evidence?
As it turns out, unlike the case with phosphorylated B6 and Riboflavin, where there is simply no advantage at all (See previous blog); the case of 5-MTHF is a bit more complex. This is how I put it in the conclusion of the folate/folic acid monograph in my most recent book (Supplementing Dietary Nutrients):
We recommend clinicians use 5-MTHF supplements, perhaps together in a B-complex product, in patients known to be homozygous (TT) for the MTHFR polymorphism. We also recommend supplementation of 5-MTHF, or 50:50 blends of folic:5-MTHF, for prenatal patients, those who are suspected of poor methylation and when using very-high-dose folate products. These suggestions should not be viewed as a recommendation to avoid folic acid in these subjects, as current data suggests neither lack of efficacy or harm when using folic acid in these subjects. There is, however, sufficient data and mechanisms to prefer 5-MTHF in these patient types. Folic acid is adequate for multivitamins intended for the average healthy population, especially where cost may hinder the use of necessary supplementation.
Here I would like to address some of the questions I often get when I discuss this topic and the above recommendation with clinicians:
Why isn’t there a bigger difference in the clinical benefit between 5-MTHF and Folic acid in homozygous MTHFR TT individuals? This question stems from the fact that contrary to some expectations, the differences seen in clinical trials when using folic acid and 5-MTHF in these individuals is often, though not always, significant.
First of all, for those not up to speed on the MTHFR language- the methylenetetrahydrofolate reductase enzyme is necessary as the terminal step in producing 5-MTHF- the active form of folate. In sequencing the gene for this protein, researchers identified that some individuals had a cytosine (C) at base pair position 677 (this is the most common), and others had thymidine (T) at that position. This is often referred to as MTHFR C677T polymorphism, and causes an alanine-to-valine amino acid change at the 222 position of the protein. This small change in the protein results in less-efficient synthesis of active folate compounds—some estimates are 75% less efficient. About 45% of individuals in the U.S. are homozygous for the normal variant (677CC), but about 10% may be homozygous for the other variant (677TT) and others (~45%) heterozygous (677CT) with both gene variants. These heterozygous, and especially homozygous TT, individuals often have noticeably less-efficient methylation, accounting for higher risk for certain diseases and a higher need for folate supplementation.
One of the most comprehensive reviews comparing these two folate compounds concludes that “Studies comparing L-5-methyl-THF and folic acid have found that the two compounds have comparable physiological activity, bioavailability and absorption at equimolar doses. Bioavailability studies have provided strong evidence that L-5-methyl-THF is at least as effective as folic acid in improving folate status, as measured by blood concentrations of folate and by functional indicators of folate status, such as plasma homocysteine.”
While there is documented clinical benefit to using 5-MTHF in patients with the TT polymorphism (this is now a common test available from many laboratories), I believe the main reason there is not always a measurable clinical difference between 5-MTHF and folic acid is that oral dosing of 5-MTHF only compensates for the initial methylation of that “dose” of folate- which is only a small portion of the total body folate pool. Meaning, that whatever benefit in the increased 5-MTHF levels is derived from consuming 5-MTHF directly (as opposed to consuming folic acid)- it is still a small proportion of the total amount of folate already in the body, most of which would need to be re-methylated within the cells. While I still recommend the use of 5-MTHF in 677TT homozygous individuals or those suspected of poor methylation, the notion that folic acid does not work at all in these individuals is refuted by nearly every trial published. Even when the 5-MTHF is statistically better than the folic acid (as it often is)- both agents are significantly (statistically and clinically) better than placebo at raising RBC folate levels and reducing homocysteine levels. If they were the same cost to the user- we might choose 5-MTHF all the time, but there is a big difference in cost between these two (see below).
5-MTHF is natural and folic acid is synthetic- right?
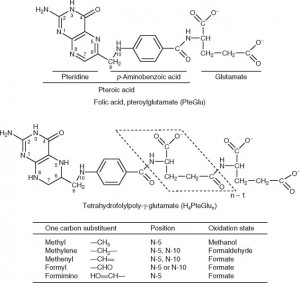
Again, this is another common misunderstanding about folates. While it is true that natural dietary folates are often in the 5-methyltetrahydrofolate form- most of these dietary folates are also naturally polyglutamyl molecules. Folic acid, on the other hand, is a monoglutamate molecule that is fully oxidized (no hydrogens attached to the ring structures). Natural folates are usually partially or fully reduced (dihydro- or tetrahydrofolate, respectively), and are substituted at the 5 or 5,10 positions with methyl or other groups (see figure below) and have up to 7 or so glutamate molecules attached (polyglutamates).
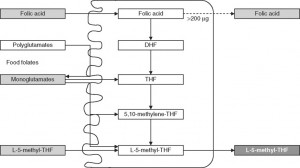
Since only monoglutamate folates are transported into the body, all dietary polyglutamyl folates must by enzymatically deconjugated to their monoglutamyl form prior to absorption in humans. This deconjugation is performed by pteroylpolyglutamate hydrolase enzymes secreted from the brush border of the jejunum. Once inside the cell, further absorption is dependent upon the reduction and methylation steps needed to form 5-MTHF as seen in the figure below (some folic acid can passively absorb and can exist as “unmetabolized” folic acid- an issue that will be discussed below)
Due to various factors, especially the need to deconjugate polyglutamyl dietary folates, synthetic folic acid is usually considered to have approximately twice the bioavailability as dietary folates. When the Food and Nutrition Board set the Dietary Reference Intake (DRI) levels for folate (using dietary folate equivalents- DFEs) they determined that 1 mcg of folate from the diet is equal to 1 DFE, while 1 mcg of folic acid in supplements was equal to 2 DFE. When folic acid is added to foods via fortification, 1 mcg is equal to 1.7 DFE (this takes into account some anti-folate activities in foods).
What about 5-MTHF from supplements? The 5-MTHF used in medical foods and in dietary supplements (either the calcium or glucosamine salt forms) are not derived from “natural sources” and are not, as some describe them- true dietary folates. These compounds would be considered bio-equivalent (or bio-identical) synthetic analogs (5-MTHF, when naturally found in foods, is mostly in a polyglutamyl, not monoglutamyl form). Commercially available 5-MTHF is organically synthesized using folic acid as a starting material. After reduction to tetrahydrofolate and methylation- a racemic mixture (R,S) of a monoglutamyl 5-MTHF is formed. Crystallization and separation of the two stereoisomers allows for a purified S-form, which is then stabilized using calcium or glucosamine ions (the “S” describes the specific stereochemistry at the #6 carbon; this is often also called the “L” form due to the way this isomer reflects light). This process results in a raw material that is about 200 times more expensive than an equimolar amount of folic acid.
The arguments against folic acid- How strong is it?
Before getting into the details here, it is important to recall that purified 5-MTHF ingredients have only been available for about a decade or so (calcium salt) and only more widely available to use in a variety of formulas for about five years (when the glucosamine salt became available). This means that most of what we know about folates (positive and negative) have been performed using folic acid and the data using 5-MTHF alone or in comparison to folic acid is still being researched.
Even so, in addition to the need to convert folic acid to 5-MTHF already discussed above, there are other reasons often cited for avoiding the use of folic acid.
1. Folic acid can mask (not cause) a vitamin B12 deficiency. Large doses of folic acid, typically more than 5 mg, given to an individual with an undiagnosed vitamin B12 deficiency could correct the symptoms of megaloblastic anemia without correcting the underlying vitamin B12 deficiency, leaving the individual at risk of developing irreversible neurologic damage. While definitive data is lacking, there appears to be some evidence that the use of 5-MTHF use is less likely to mask these B12 deficiency symptoms. However, since most functional medicine clinicians are aware of (and test for) vitamin B12 deficiency and most high dose folic acid and many 5-MTHF products are formulated with added vitamin B12- the issue of folate masking is rarely seen in the clinic using high dose folate therapies. We always suggest 500-2000 mcg of vitamin B12 should be included in any product containing 800 mcg or more of folic acid or 5-MTHF.
2. Un-metabolized folic acid. As we mentioned, while nearly all of the folic acid consumed orally will be converted to 5-MTHF prior to circulation, some folic acid does passively absorb prior to conversion. While it is now common for researchers to look for unmetabolized folic acid levels in the serum of folic acid-supplemented individuals, there is no clear evidence of a demonstrable negative consequence for these elevated levels or a proposed mechanism involving negative outcomes. The paper below covers most of the issues related to unmetabolized folic acid.
So our position is that while the use of 5-MTHF may have some benefits over the use of folic acid, the much higher cost of pure 5-MTHF over folic acid requires that we reserve its use when it will be more likely to add value to the user. Since the data do not suggest folic acid to be harmful (compared to 5-MTHF) and clinically useful in most situations- the use of 5-MTHF should be considered mostly for those individuals with MTHFR polymorphisms that result in lower methylated folate. When using either folate compound above 800 mcg/day, additional vitamin B12 should be added to the regimen or formula.
If you find this information helpful, you may be interested in our new book- Supplementing Dietary Nutrients- A Guide for Healthcare Professionals.
If you want to know when Dr. Guilliams’ posts future blogs or when a new whitepaper is available, “Like” us on our Facebook page.
To get Dr. Guilliams’ earlier book, The Original Prescription– you can purchase directly from the Point Institute or purchase on Amazon.
And don’t forget to sign up for our blog at the bottom of this page!
New Book at the Printer – Soon Available!
It has been a little while since I have been able to post a new message here on the Point Institute website; I have been busy editing and proofing our latest project, which, I am pleased to announce, is finally at the printer and will be available shortly.
Supplementing Dietary Nutrients: A Guide for Healthcare Professionals
This new guidebook was written as the first in our “Standard – Roadmap Series” (more to come later on this) focusing on the use of nutrient ingredients for dietary supplementation. Using the same sort of approach we have used in developing the Standard monographs since 1997, this guidebook can be used as a training manual or textbook and kept handy for its monograph information on over 30 nutrients. To see a bit more about the details of the book, go here.
As the book is slated to arrive in a few weeks, we are making it available first on our website, and will distribute it here for 20% off the cover price until May 15th (including free shipping in the US). If you want to get your copy, click the Buy Now button below:
They will be sent out as soon as they arrive.
A tremendous amount of work went into this guidebook and we have received great feedback from everyone who has seen the manuscript in progress. We are certain that anyone making supplemental nutrient recommendations within a healthcare setting will want to have this on their shelves.
Krill Oil: How its latest “success” proves it to be an epic failure…again
Those familiar with our work know that we have spent quite a bit of time evaluating the therapeutic outcomes of marine-derived omega-3 fatty acids. Our recent review of the topic has been downloaded and widely circulated amongst healthcare providers and the general public, worldwide. In that review, we covered the types of fish used, how fish oil is made, sustainability issues, bioavailability differences, quality control concerns and much more; including the research comparing omega-3 fatty acid from fish oil and krill oil. You can get the article as a PDF file here.
Just a month after publishing our paper online, a few more studies comparing fish oil and krill oil were published that initially appeared to suggest that omega-3 fatty acids from krill oil may indeed have a slightly better bioavailability than those from fish oil and/or had triglyceride lowering effects similar to fish oil; but after only a month of scrutiny, these studies are exposed as epic failures of how marketing-driven research leads to bad science and confusing outcomes.
First- as a brief review for those who haven’t read our whitepaper. Our position was that the EPA and DHA in krill oil should function in much the same way as EPA and DHA from fish oil- assuming equal amounts of the fatty acid become bioavailable after consumption. The typical claims made by the marketers of krill oil is that, because krill oil omega-3s are delivered as phospholipids (PL, rather than triglycerides), they will have (or have been shown to have) higher bioavailability than fish oil omega-3s. Our whitepaper clearly shows that the studies used by marketers to “prove” such assertions are either not clinically or statistically significant; or are not appropriately designed to make such comparisons. However, the biggest issue is not their failure to prove better bioavailability, or the fact that krill oil appears to be nearly ¼ free fatty acids upon analysis (not all PL as claimed); but the fact that commercially available krill oil products are extremely low in EPA and DHA, while still costing much more than fish oil products (containing much more EPA and DHA). In fact, in the only trial comparing equivalent doses, researchers needed to use 14 krill oil capsules to get the same amount of EPA and DHA as 4 capsules of fish oil.
This is where the first of the new studies fits in. Published in December of 2013 in the open access Lipids in Health and Disease, this paper has such a hopeful title: Enhanced increase of omega-3 index in healthy individuals with response to 4-week n-3 fatty acid supplementation from krill versus fish oil [Free Download]. The cross-over designed trial appears to compare an equal amount of EPA and DHA from krill oil and fish oil (and a corn oil placebo); and indeed reports a higher increase in the omega-3 index (the percent of EPA and DHA within RBC phospholipids) during the time subjects were taking krill (compared to fish oil); although both fish and krill oil were better than placebo. They report that the various oils were provided in six- 500 mg capsules (3 with breakfast, 3 with dinner); describing the fish oil as a “TG 18/12” oil. Going one step further; they analyze and report the fatty acid composition of each of the three oil products; and this is where things get fishy.
They claim the fish oil to which they compared the krill oil was a TG 18/12, which is the usual designation for un-concentrated fish body oil providing 180 mg of EPA and 120 mg of DHA per 1000 mg of oil. However, their fatty acid composition lists a very unusual fatty acid profile for this fish oil: including 32% linoleic acid- an omega-6 fatty acid. Normally, fish oil contains about 2-3% omega-6 fatty acids; so what is going on with this oil? Well, we were not the only ones to wonder about this. In early January of 2014, a commentary of the above trial was also published in the same journal, asking about the strange fatty acid profile of this fish oil, along with a few other points of contention. You can find that Commentary Here.
Incredibly, the authors of the original paper explained it this way in their rebuttal [Found Here]: Our primary objective was to compare effects of consumption of same amount of n-3 fatty acids from krill or fish oil. When designing a double blinded placebo controlled randomised cross over trial, it was felt that the amounts of treatment products as well as the bioactives of interest be maintained consistent across different interventions. However, the n-3 PUFA content of the krill oil fell below that of fish oil. In order to match the concentrations of n-3 PUFA and volumes between krill and fish oil, the fish oil was diluted with the placebo, corn oil at a ratio of 1.3:1.0.[Emphasis added] Yes, you read that correctly. They used the lowest dose of fish oil they could find (one shown to have lower bioavailability than the concentrated TG forms) and still needed to dilute it with corn oil so they could reduce its omega-3 content for a head-to-head comparison to krill oil. The authors also admit: We agree that we could have included the information about dilution of fish oil in the original manuscript itself. While we will avoid the obvious question about motive usually entertained when a manufacturer of krill is involved in such a study; this begs the question of the type of expertise used in the peer-reviewing process that missed the obvious questions about the fatty acid profile of the fish oil.
This report, with the commentary and rebuttal, only solidifies our view that krill oil simply cannot deliver a cost-effective payload of EPA and DHA to be considered as a therapeutic alternative to fish oil. Krill oil products do not even have the amount of EPA and DHA found in the lowest concentrations of fish oil, while their cost is sometimes double or triple the same. From a therapeutic standpoint, concentrated TG forms can deliver 4-8 times more EPA and DHA per capsule, at an affordable price.
This brings us to the second paper, published in the February 2014 edition of Nutrition Research. Again, the title of this article (written by scientist employed by the manufacturer of the krill product used) was deceptively hopeful: Krill oil supplementation lowers serum triglycerides without increasing low-density lipoprotein cholesterol in adults with borderline high or high triglyceride levels. Unfortunately, the data proved to be anything but a straight-forward TG-lowering effect from krill oil- although their analysis is so flawed that it almost defies explanation.
The study was designed much like TG-lowering studies of fish oil. Three-hundred patients with high triglycerides were recruited and given placebo or 3 to 4 grams of krill oil providing 0, 100, 200, 400 or 800 mg of EPA/DHA over 12 weeks. Blood lipids and omega-3 index were measured at baseline, six weeks and 12-weeks after consuming the krill products. The data speaks for itself; after 12-weeks of krill oil consumption the change in TG levels in these individuals with a mean TG at baseline=231 was as follows: Placebo (+3.9%), 100 mg (-10%), 200 mg (-3.8%), 400 mg (-6.7%), 800 mg (+0.9%)- none of these reached statistical significance. The authors claim that the lack of efficacy and dose-response was due to the overwhelming intra-individual TG measurements and high standard deviation- making it impossible to measure fasting TG as an outcome. How then, with these numbers (even showing an increase in TG using 800 mg of EPA and DHA) were they able to declare a TG-lowering effect in the title?
The reviewers of the paper allowed these authors to circumvent the “limitations” of the study and use “an explorative data analysis approach to increase the statistical power of the study.” In essence what they did was to pool together all the doses, including the 6-week data points which happened to be better for nearly all the doses, and analyzed the data as if a theoretical average EPA/DHA content of 385 mg was given to all the subjects. The authors then boldly declare that “Relative to subjects in the placebo group, those administered krill oil had a statistically significant calculated reduction in serum TG levels of 10.2%.” Even if we accepted this flawed explorative data analysis, this data showed only a 6.3% reduction from baseline TG levels- a level that even if achieved in this study, represents a small clinical difference. In contrast, fish oil studies routinely see drops in TG (from baseline) of >25%, show clear dose-response and are maintained or even continue to improve between 6 and 12 weeks.
The fact that such a flawed study that failed to reach any statistical-significant reductions in TG based on the primary objective (12-weeks) and initial statistical plan was permitted to use statistical manipulation to imply a positive outcome is incomprehensible. Clinicians and patients would read the title and abstract of this paper thinking that krill oil was able to reduce TG levels in these subjects- when in fact, at the end of 12 weeks the data shows that it did not. This paper should be retracted, rewritten to describe it as a failure to meet its TG-lowering objective and republished.
While I am certain the marketing departments of krill manufacturers and distributors are eager to share with you their “latest success stories”- now you have the rest of story- revealing krill oil’s epic failure as a therapeutic contender in the omega-3 world.
[Dr. Guilliams discussed this and many more issues related to fish oil (from the whitepaper) in a discussion with Dr. Hoffman’s on his Intelligent Medicine podcast. Download and Listen Here.]
If you want to know when Dr. Guilliams’ posts future blogs or when a new whitepaper is available, “Like” us on our Facebook page.
To get Dr. Guilliams’ book The Original Prescription– you can purchase directly from the Point Institute or purchase on Amazon.
Sign up for our blog at the bottom of this page!
Dr. Guilliams’ multivitamin article in the latest IMCJ
Dr. Guilliams’ recent blog on multivitamins was picked up by Integrative Medicine: A Clinician’s Journal (IMCJ) – read it here.
Isomers, Enantiomers and Activated Forms: Part 1 – When does the exact form of a nutrient really matter?
Over the past six months, I have been working on a big project (soon to be announced) evaluating a whole host of assumptions and presumptions concerning nutrients and their various forms. Natural vs. Synthetic, isolates vs. complexes, “activated” forms and chiral enantiomers. I hope to use a few separate blogs to help clinicians, researchers and interested nutrient aficionados understand when these differences appear to matter (at least in a way that can be measured). By the way, we will also try to make sense of all this d,l and R,S confusion that only seems to make sense to organic chemists.
Some of the ones we will be covering over the next few blogs include: natural vitamin E (d-alpha) vs. synthetic vitamin E (d,l-alpha tocopherol); alpha vs. beta, gamma, delta tocopherols; vitamin K1 vs. K2; phosphorylated B6 and riboflavin forms, methylated folate and B12, R-lipoic acid vs R,S-Lipoic acid, Co-Q10 (ubiquinone) vs. ubiquinol and a few others that crop up now and again.
Knowing that these topics can be a bit controversial, I want to start with one that is sure to stir the pot a little, but is fairly straightforward to explain: phosphorylated B-vitamins. It is quite common, especially here in the US, to hear well-regarded clinicians recommend the use of activated B-vitamins from the podium of prestigious integrative medicine conferences. Along with methyl-folate and B12, this list usually includes pyridoxal-5-phophate (P5P) and riboflavin-5-phosphate (R5P). But is there any evidence to suggest that either of these forms is better than their “inactive” counterparts? In fact, there is not; and the reason is shamefully obvious: both P5P and R5P must be de-phosphorylated prior to transport into the body.
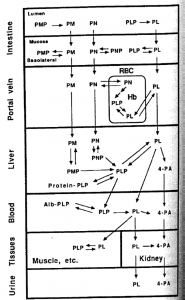
Food sources of vitamin B6 are found in all three forms (pyridoxine (PN), pyridoxamine (PM), pyridoxal (PL)), as well as each of their phosphorylated and glucoside-conjugated forms. All phosphorylated compounds are hydrolyzed into their respective un-phosphorylated form prior to absorption, after which in vivo conversion allows each to act as vitamin precursors. Phosphorylation of the coenzyme form occurs, as needed, in the liver. (See the figure from pg 176 of Present Knowledge in Nutrition-7th edition). Note that in each case the form of B6 is transported from one tissue to another, the phosphate portion is removed.
The same phenomenon exists for riboflavin and riboflavin-5-phopshate. Riboflavin is naturally found in foods as either free riboflavin or one of its co-enzyme derivatives (FAD/FMN). The bioavailability of these compounds is similar since each form is hydrolyzed to free riboflavin prior to transport into the body. The similar pharmacokinetics of oral R5P (and its need for de-phosphorylation) compared to pyridoxine HCl have been known for over 45 years! So why is this so important? If they are the same, why bother with it all? Well first let’s also add to our discussion that the raw material cost (both in dollars and energy) is greater using these forms (about 3-times higher for R5P and about 7-times higher for P5P) and they are no more “natural” since each is chemically synthesized from either free riboflavin or pyridoxine HCl, respectively.
Beyond the cost difference for the use of ingredient with no additional benefits, there are two other concerns that I would like to raise before closing. The first is that the blanket notion that consuming the “active” form of a vitamin will somehow improve the overall benefit of an oral supplement simply teaches the wrong view of vitamin physiology. All vitamins function in the body by a constant conversion between various active and inactive forms. In many cases, active vitamins are converted to their inactive forms (intentionally) by specific enzymes in order to transport vitamins from one part of the body to another. Inactivating the vitamin prevents it from inappropriately reacting prior to reaching the target tissue. Secondly, when such broad statements about “activated” vitamins are made, it makes it more difficult to explain the true differences between certain nutrients, when proven differences actually exists (many of which we will cover in future blogs).
I am fairly certain that most clinicians, nutritionist, dieticians and even supplement manufacturers are unaware of this information (even though it is in all the textbooks); so no need to panic. In this case, these phosphorylated forms are not necessarily less clinically effective, they are just less cost effective ways to deliver the same nutrient.
• Vitamin B6 page- Linus Pauling Institute website: http://lpi.oregonstate.edu/infocenter/vitamins/vitaminB6/
• Office of Dietary Supplements- National Institutes of Health- Health Professional Information. http://ods.od.nih.gov/factsheets/VitaminB6-HealthProfessional/
• Said HM. Intestinal absorption of water-soluble vitamins in health and disease. Biochem J. 2011 Aug 1;437(3):357-72
• Waldmann A, Dörr B, Koschizke JW, Leitzmann C, Hahn A. Dietary intake of vitamin B6 and concentration of vitamin B6 in blood samples of German vegans. Public Health Nutr. 2006 Sep;9(6):779-84.
• Gregory JF 3rd. Bioavailability of vitamin B-6. Eur J Clin Nutr. 1997 Jan;51 Suppl 1:S43-8.
• Riboflavin Page- Linus Pauling Website: http://lpi.oregonstate.edu/infocenter/vitamins/riboflavin/
• Bates CJ. Bioavailability of riboflavin. Eur J Clin Nutr. 1997 Jan;51 Suppl 1:S38-42.
• Jusko WJ, Levy G. Absorption, metabolism, and excretion of riboflavin-5′-phosphate in man. J Pharm Sci. 1967 Jan;56(1):58-62.
If you want to know when Dr. Guilliams posts future blogs or when a new whitepaper is available, “Like” us on our Facebook page. To get Dr. Guilliams’ book The Original Prescription– you can purchase directly from the Point Institute or purchase on Amazon.